Agencies, 08 May: World Health Organization (WHO) para May 7, 2021 te China’s Sinopharm COVID-19 dawai toh emergency use nemite manishe. Sinopharm dawai toh etiya UN-backed COVAX vaccine distribution scheme te melaishe.
Etu khobor toh WHO chief Tedros Adhanom Ghebreyesus para press conference samai janaideya laga ase. Etu para etu dawai toh WHO para safety, efficacy aro quality nemite validation powa sixth dawai hoise.
WHO para Pfizer/BioNTech, AstraZeneca, Johnson and Johnson, aro Moderna nemite mana be mani kena ase hoilebe Sinopharm, tuita-dose dawai nemite manibole deri howa toh data deri howa nemite ase.
WHO chief Tedros para be Strategic Advisory Group of Experts on Immunisation (SAGE) para Sinopharm vaccine te data thaka opor te shaise aro two-dose schedule toh 18 omar opor khan ke debole nemite recommend korise koise.
Sinopharm dawai opor te khobor:
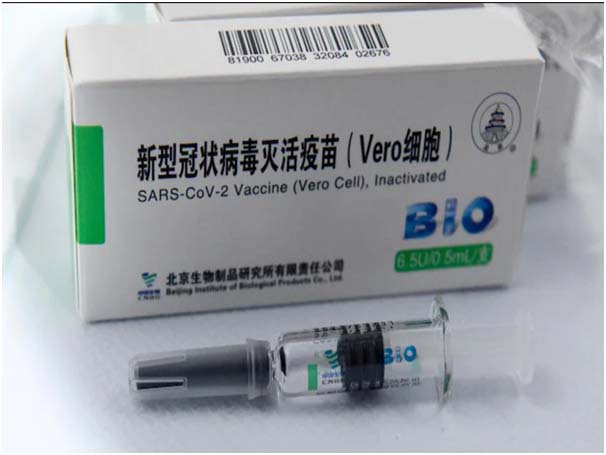
Sinopharm dawai toh Beijing Bio-Institute of Biological Products Co Ltd (China National Biotec Group (CNBG) laga ekta subsidiary) para bona laga ase.
Etu toh two-dose vaccine ase. Etu dawai toh sosta para rakhibole paribo aro low-resource settings nemite ektum thek ase.
Etu toh first dawai ase vial monitor logote aha aro dawai laga color toh heat te putli hobo.
Symptomatic cases nemite efficacy rate toh 79 percent te estimate korise.









Add Comment